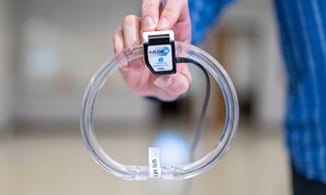
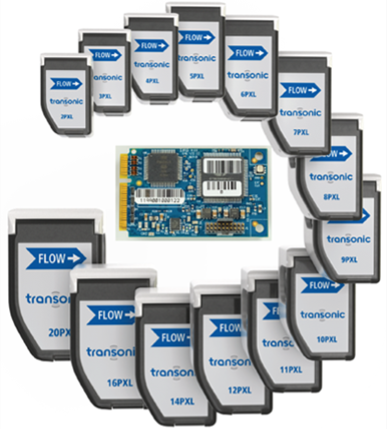
With our Transonic-Inside™ Development Program, you'll gain access to experts in Engineering, R&D, and Clinical who will guide you every step of the way. We have development kits to get you started quickly and a flexible gate development process that will be tailored to your needs.